The yeast genome-wide knockout library is a set of mutant yeasts with defects in the functions of specific yeast genes and consists of mutants targeting approximately 98.5% of the genes of the entire fission yeast (S. pombe) genome. Gene function-deficient variants were created by inserting an antibiotic resistance gene and barcode instead of the target gene using the homologous gene recombination method. The process is as follows:
1. Target gene selection: Identify target genes in the yeast genome (Shizosaccharomyces pombe).
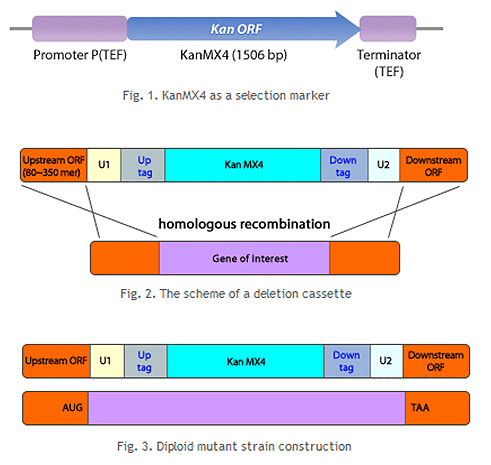
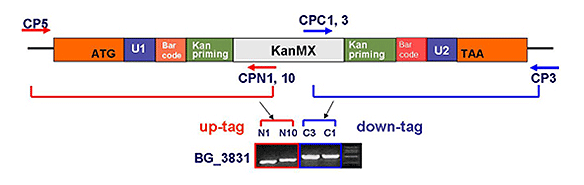
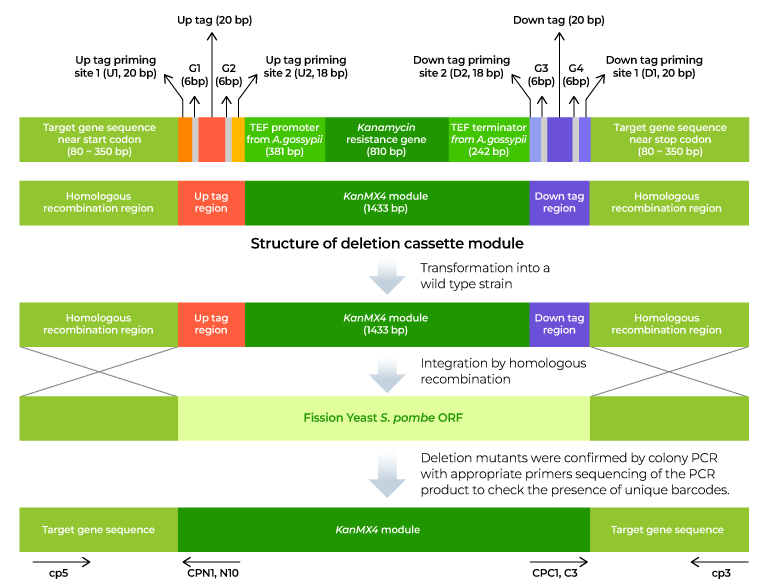
2. Preparation of DNA cassette: Prepare a DNA cassette to be recombined with the yeast target gene through homologous recombination. The DNA cassette contains a recombinant target gene ORF (open reading frame) on both flanks, a geneticin (G418) resistance gene (KanMX) cassette for selection of knockout strains, and a unique barcode for identification of each variant.
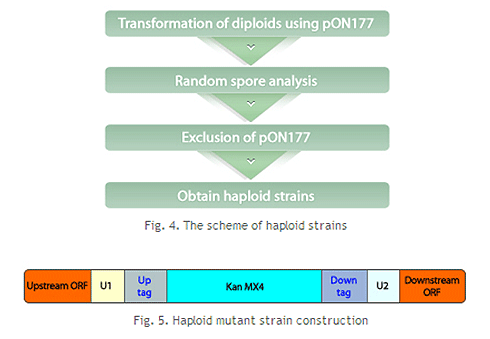
3. Yeast transformation: Transformation technology is utilized to introduce the DNA cassette into yeast cells. The DNA cassette entered into the yeast cell recognizes the homologous sequence of the flanking region of the target gene on the genome and is then inserted into the corresponding location. The target gene ORF is removed, preventing its function in yeast. Additionally, yeast cells with the DNA cassette inserted at the same time become G418 resistant.
4. Selection of knockout strains: Transformed yeast is grown in medium containing G418 to select only knockout yeast. This process is repeated for all target genes, and finally, all knockout strains are prepared as one set and provided as a library.




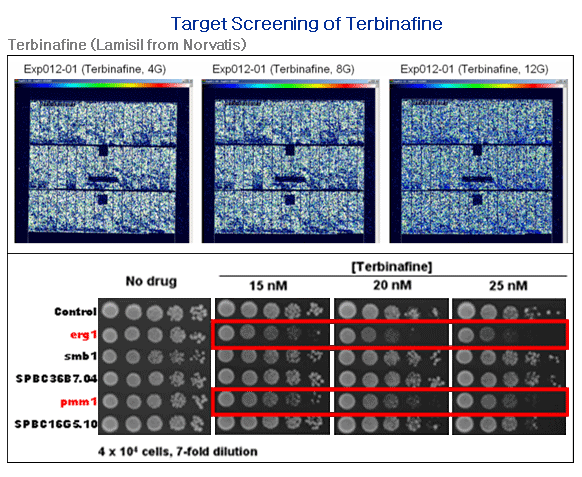
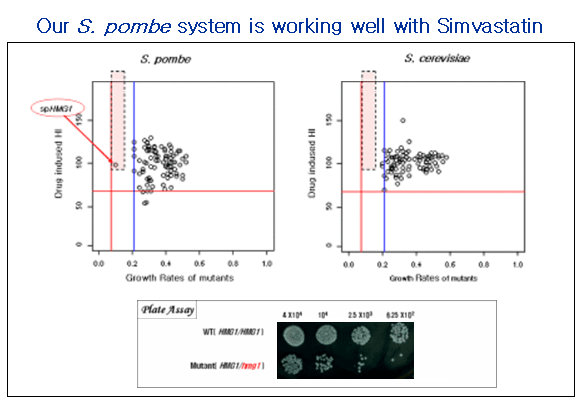
 Application #1. Target screening for anti-fungal agents
Application #1. Target screening for anti-fungal agents