We use cookies to give you the best online experience. By using our website you agree to our use of cookies in accordance with our Cookie Policy
AccuPower® HIV-1 Quantitative RT-PCR Kit
AccuPower® HIV-1 Quantitative RT-PCR Kit is an in vitro diagnostic kit designed for the quantification of HIV-1 RNA in EDTA-plasma samples using Real-Time PCR.
AccuPower® HIV-1 Quantitative RT-PCR Kit is an in vitro diagnostic assay designed for the quantitative detection of HIV-1 RNA in EDTA plasma samples using real-time RT-PCR. Monitoring HIV-1 viral load is essential for establishing treatment strategies and evaluating therapeutic response in infected patients.
The kit provides sensitive and reliable quantification of HIV-1 RNA across diverse subtypes and has been internationally validated through regulatory certifications:
-
Cat. No. HIV-1111: WHO Prequalified
-
Cat. No. HIV-1211: CE List A certified
With these certifications, the AccuPower® HIV-1 Quantitative RT-PCR Kit is recognized for both global health program use (WHO PQ) and clinical diagnostic applications in Europe (CE-IVD List A).
The kit is intended for use on the ExiStation™ Universal Molecular Diagnostic System.
The standard workflow includes the AccuLoader™, a guided sample loader for contamination control.
Features and Benefits
- HIV-1 Subtype Detection: HIV-1 subtype group M (A, B, C, D, AE, F, AG, G, H and several CRFs), N and O are detectable with high sensitivity.
- Signature Convenience: One-step RT-PCR premix type. All components for the assay are contained within a tube.
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary Dual HotStart technology accomplishes high sensitivity and specificity
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results
- High Quality: Manufactured under strict quality control guidelines, and the product is certified WHO Prequalification for reliable and globally recognized diagnostics
Specifications
| Specimen Type | EDTA-plasma |
| Kit Contents | PCR Premix, SPC 1~5, LPC, HPC, NTC, SL buffer, DEPC D.W. |
| Instrumentation | ExiStation™ Universal Molecular Diagnostic System |
| Tests | 96 |
Performances
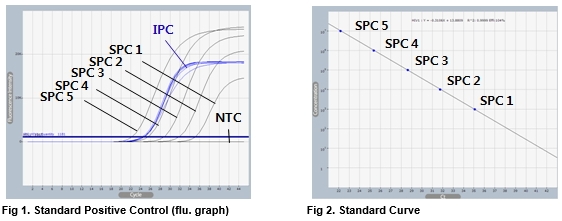
AccuPower® HIV-1 Quantitative RT-PCR Kit includes serially diluted Standard Positive Control (SPC) 1~5 for the quantification of HIV-1.
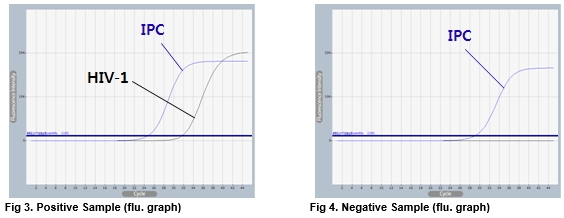
AccuPower® HIV-1 Quantitative RT-PCR Kit test results using clinical samples. The kit employs IPC in all wells to confirm correct PCR amplification.
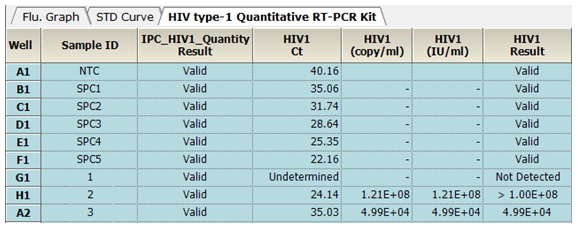
User centric ExiStation Manager software automatically analyzes the test results based upon the Ct (threshold cycle) value.
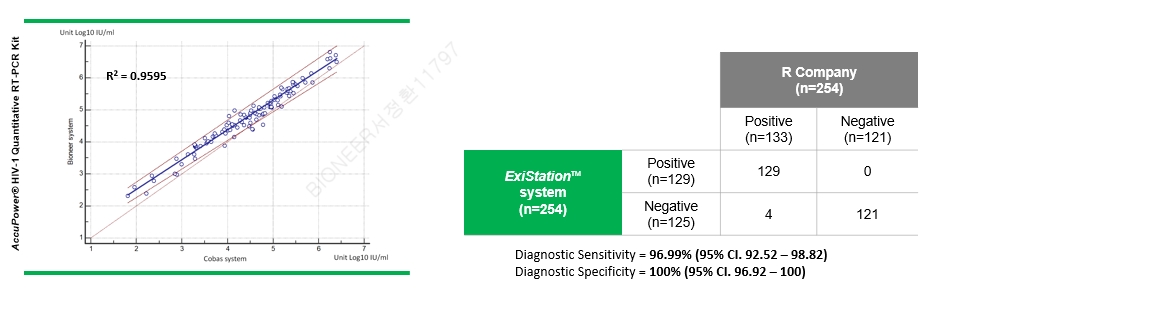
The clinicla evaluation of AccuPower® HIV-1 Quantitative RT-PCR Kit in Cerba Specimen Services(France). Total 254 samples(234 ea HIV-1 positive/negative European specimens & 20 additional samples) were used.
Ordering Information
| Cat. No. | Product Description | Certification |
| HIV-1211 | AccuPower® HIV-1 Quantitative RT-PCR Kit (96 tests) | CE List A |
| HIV-1111 | AccuPower® HIV-1 Quantitative RT-PCR Kit (96 tests) | WHO PQ |
Related Products
| Cat. No. | Instrument |
| A-5251 | AccuLoader™ |
| A-2060 | Exicycler™ 96 Real-Time Quantitative Thermal Block |
| A-2200 | ExiStation™ Universal Molecular Diagnostic System |
| A-5050 | ExiPrep™16 Dx Automated Nucleic Acid Extraction System (16 tests) |
| Cat. No. | Nucleic Acid Extraction Kit |
| K-4471 | ExiPrep™ Dx Viral DNA/RNA Kit (96 tests) |
| K-4473 | ExiPrep™ Dx Viral RNA Kit (96 tests) |
Quality Assurance
Bioneer is the holder of Quality Management System Certificates for the following standards.
Contact Us
E-mail : sales@bioneer.com




