We use cookies to give you the best online experience. By using our website you agree to our use of cookies in accordance with our Cookie Policy
AccuPower® MTB & NTM Real-Time PCR Kit

Tuberculosis (TB) is caused by a bacterium called M. tuberculosis. The bacteria usually attack the lungs, but TB bacteria can attack any part of the body such as the kidney, spine, and brain. It can affect mainly the lungs (pulmonary TB). Non-tuberculous mycobacteria (NTM) are a group of bacteria, normally found in soil and water and some domestic and wild animals that can cause severe lung disease.
Diagnosing MTB, NTM can be difficult because symptoms may be similar to other lung conditions. Recently, molecular diagnostic methods, including PCR or real-time PCR, provide higher sensitivity and specificity than other conventional diagnostic methods.
Features and Benefits
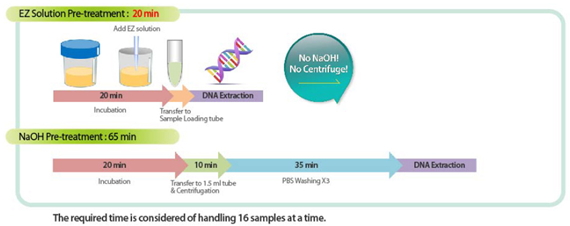
- Signature Convenience: Simultaneous detection of MTB and NTM (57 species). Simplified procedure of sputum pretreatment with EZ Solution.
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
- Sputum Pre-treatment: EZ Solution vs NaOH
Specifications
| Specimen Type | Sputum, bronchoalveolar lavage (BAL), urine |
| Kit Contents | PCR Premix, PC, IPC, NTC, DEPC D.W. |
| Instrumentation | ExiStation™ ™ Universal Molecular Diagnostic System or Exicycler™ 96 |
| Tests | 96 |
Performances
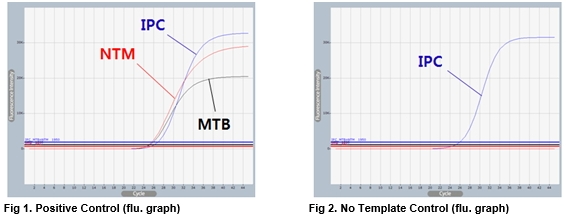
AccuPower® MTB&NTM Real-Time PCR Kit includes Positive Control (PC), No Template Control (NTC) and Internal Positive Control (IPC) for accurate and reliable diagnosis of MTB and NTM.
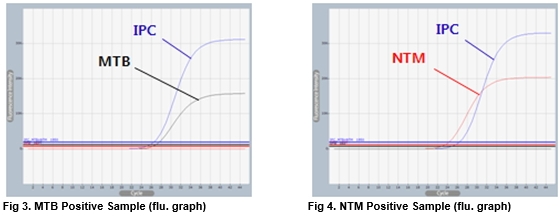
AccuPower® MTB&NTM Real-Time PCR Kit test results using clinical samples. The kit employs IPC in all wells to confirm correct PCR amplification.
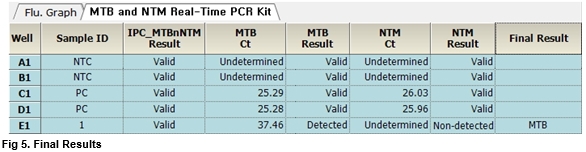
User centric ExiStation Manager software or ExiDiagnosis software automatically analyzes the test results based upon the Ct (threshold cycle) value.
Ordering Information
| Cat. No. | Product Description |
| MTN-1111 | AccuPower® MTB&NTM Real-Time PCR Kit (96 tests) |
Related Products
| Cat. No. | MDx Diagnostic Kit |
| MTB-1111 | AccuPower® MTB Real-Time PCR Kit (96 tests) |
| NTM-1111 | AccuPower® NTM Real-Time PCR Kit (96 tests) |
| TBMDR-1111 | AccuPower® TB&MDR Real-Time PCR Kit (48 tests) |
| Cat. No. | Instrument |
| A-2060 | Exicycler™ 96 Real-Time Quantitative Thermal Block |
| A-2200 | ExiStation™ Universal Molecular Diagnostic System |
| A-5050 | ExiPrep™16 Dx Automated Nucleic Acid Extraction System (16 tests) |
| Cat. No. | Nucleic Acid Extraction Kit |
| K-4414 | ExiPrep™ Dx Bacteria Genomic DNA Kit (96 tests) |
| K-4418 | ExiPrep™ Dx Mycobacteria Genomic DNA Kit (96 tests) |
Quality Assurance
Bioneer is the holder of Quality Management System Certificates for the following standards.
Contact Us
E-mail : sales@bioneer.com




