We use cookies to give you the best online experience. By using our website you agree to our use of cookies in accordance with our Cookie Policy
AccuPower® HPV 16&18 Real-Time PCR Kit
AccuPower® HPV 16&18 Real-Time PCR Kit is an in vitro diagnostic kit designed for the simultaneous detection of Human papillomavirus (HPV) type 16 and 18 DNA in human cervical swab samples through real-time PCR.
The global incidence and death from cervical cancer rank the second and third highest respectively among women. The main cause of cervical cancer is Human papillomavirus (HPV) in the cervix. There are multiple types of HPV, sometimes called "low risk" and "high risk" types. Low risk types cause warts and high risk types can cause lesions or cancer. According to the study with 196 patients in cervical adenocarcinoma, HPV genotype 18 is responsible for cervical cancer in 54.2% of the patients; HPV 16, in 44.1%.
Features and Benefits
- Signature Convenience: All components for the assay are contained within a tube.
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
Specifications
| Specimen Type | Cervical swab |
| Kit Contents | PCR Premix, PC, IPC, NTC, DEPC D.W. |
| Instrumentation | ExiStation™ Universal Molecular Diagnostic System or Exicycler™ 96 |
| Tests | 96 |
Performances
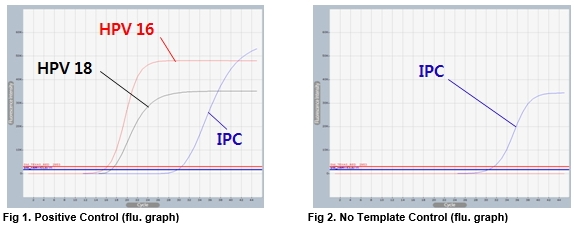
AccuPower® HPV 16&18 Real-Time PCR Kit includes Positive Control (PC), No Template Control (NTC) and Internal Positive Control (IPC) for accurate and reliable diagnosis of HPV 16 and 18.
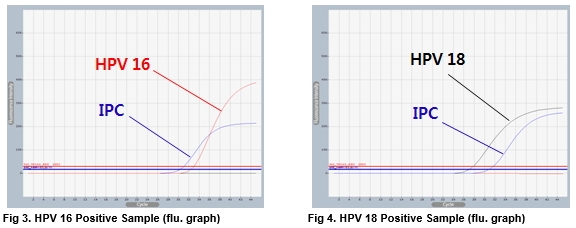
AccuPower® HPV 16&18 Real-Time PCR Kit test results using clinical samples. The kit employs IPC in all wells to confirm correct PCR amplification.
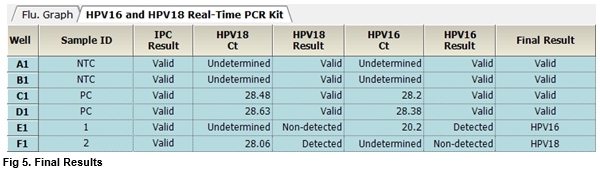
User centric ExiStation Manager software or ExiDiagnosis software automatically analyzes the test results based upon the Ct (threshold cycle) value.
Ordering Information
| Cat. No. | Product Description |
| HPM-1111 | AccuPower® HPV 16&18 Real-Time PCR Kit (96 tests) |
Related Products
| Cat. No. | Instrument |
| A-2060 | Exicycler™ 96 Real-Time Quantitative Thermal Block |
| A-2200 | ExiStation™ Universal Molecular Diagnostic System |
| A-5050 | ExiPrep™16 Dx Automated Nucleic Acid Extraction System (16 tests) |
| Cat. No. | Nucleic Acid Extraction Kit |
| K-4471 | ExiPrep™ Dx Viral DNA/RNA Kit (96 tests) |
| K-4472 | ExiPrep™ Dx Viral DNA Kit (96 tests) |
Quality Assurance
Bioneer is the holder of Quality Management System Certificates for the following standards.
Contact Us
E-mail : sales@bioneer.com



