We use cookies to give you the best online experience. By using our website you agree to our use of cookies in accordance with our Cookie Policy
AccuPower® hrHPV Genotyping & Screening Kit
Product Description
The global incidence and death rates of cervical cancer rank the second and third highest respectively among women. It adds 4,000 ~ 5,000 new cases every year and the incidence rates in young women are increasing. The main cause of cervical cancer is Human papillomavirus (HPV) that infects cervix. The normal cells lining the cervix gradually change to pre-cancerous cells and then turn into cancer by certain virus-producing proteins. The pre-cancerous transformation as a precursor of cervical cancer is referred to as cervical intraepithelial neoplasia (CIN), squamous intraepithelial lesion (SIL), or dysplasia. There are two main types of cervical cancers depending on the cancer location in the cervix: squamous cell carcinoma that occurs in the extocervix and adenocarcinoma in the endocervix.
Today, more than 200 HPV genotypes have been defined, among which 50 can infect the cervical epithelia. high risk (hrHPVs) and 14 show strong evidence of a causal link to cervical cancer. Molecular tests for HPV detection may be a good alternative to cytology for early screening because an HPV-negative result is sufficient to declare an extremely low risk of developing CIN3 or worse (CIN3+) for 5 years in contrast to a negative cytology result.
Features and Benefits
- Signature Convenience : Multiplex detection of High-risk Human papillomavirus.
- Enhanced Sensitivity & Specificity : Bioneer’s proprietary HotStart™ technology accomplishes high sensitivity and specificity.
- Remarkable Stability : Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
Specifications
| kit | AccuPower® hrHPV Genotyping & Screening Kit |
| target | High-risk Human papillomavirus |
| Sample Type | Cervical swab |
| component | PCR premix, PC, NTC, DEPC D.W., Optical sealing film150 ul |
| Instrumentation | ExiStation™, Exicycler™ 96 |
Experimental Data
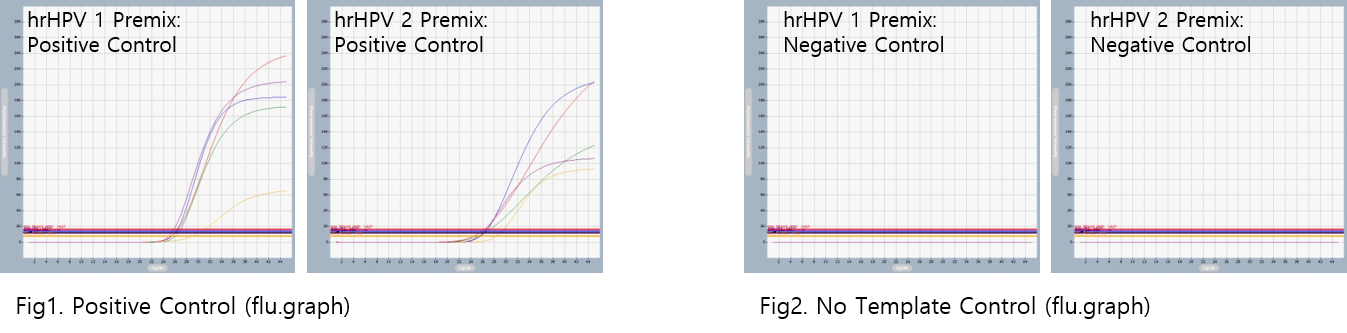
AccuPower® hrHPV Genotyping & Screening kit includes Positive Control (PC), No Template Control (NTC) for accurate and reliable diagnosis of High-risk Human papillomavirus.
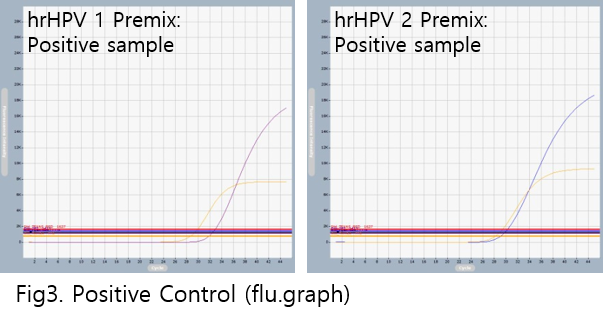
AccuPower® hrHPV Genotyping & Screening kit test results using clinical samples. The kit employs IPC in all wells to confirm correct PCR amplification.
Ordering Information
| Cat. No. | Product Description |
| HHP-1111 | AccuPower®hrHPV Genotyping & Screening Kit (48 tests) |
Related Products
| Cat. No. | Instrument |
| A-2060 | Exicycler™96 Real-Time Quantitative Thermal Block |
| A-2060-1 | |
| A-2200 | ExiStation™Universal Molecular Diagnostic System |
| A-2200-N | |
| A-5050 | ExiPrep™16Dx |
| A-5150 | ExiPrep™48Dx |
| A-5250 | Exiprep™96 Lite |
| Cat. No. | Nucleic Acid Extraction Kit |
| K-4471 | ExiPrep™Dx Viral DNA/RNA Kit (96 tests) |
| K-4472 | ExiPre™ Dx Viral DNA Kit (96 tests) |
| K-4571 | ExiPrep™ 48 Viral DNA/RNA Kit (96 tests) |
| K-4611 | ExiPrep™96 genomic DNA kit (384 tests) |
Quality Assurance
Bioneer is the holder of Quality Management System Certificates for the following standards.
Contact Us
E-mail : sales@bioneer.com




