We use cookies to give you the best online experience. By using our website you agree to our use of cookies in accordance with our Cookie Policy
AccuPower® EV Real-Time RT-PCR Kit

Enterovirus (EV) can be classified into one of five major groups, including polioviruses, echoviruses, coxsackieviruses A, coxsackieviruses B and other enteroviruses. Infected persons who become ill usually develop mild upper respiratory symptoms (a “cold”), a flu-like illness with fever and muscle aches, or an illness with rash. Rarely, a person may develop an illness that affects the heart (myocarditis) or the brain (encephalitis) or causes paralysis.
Features and Benefits
- Signature Convenience: One-step RT-PCR premix type. All components for the assay are contained within a tube. Just add sample!
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
Specifications
| Specimen Type | Stool, cerebrospinal fluid (CSF) |
| Kit Contents | PCR Premix, PC, IPC, NTC, DEPC D.W. |
| Instrumentation | Exicycler™ 96 |
| Tests | 96 |
Performances
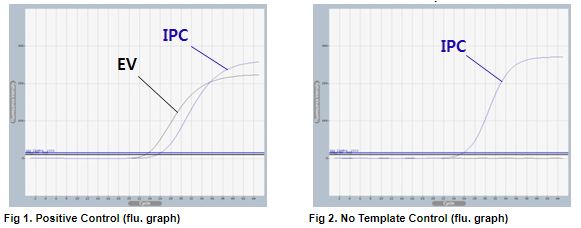
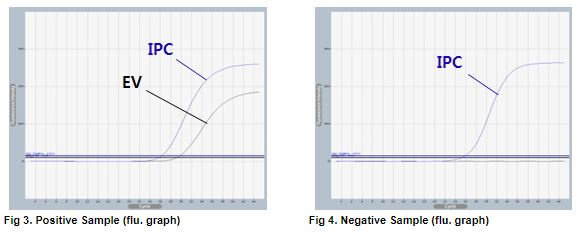
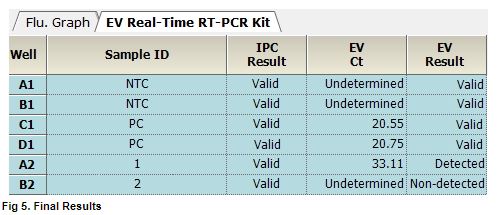
AccuPower® EV Real-Time RT-PCR Kit includes Positive Control (PC), No Template Control (NTC) and Internal Positive Control (IPC) for accurate and reliable diagnosis of enterovirus.
AccuPower® EV Real-Time RT-PCR Kit test results using clinical samples. The kit employs IPC in all wells to confirm correct PCR amplification.
User centric ExiDiagnosis software automatically analyzes the test results based upon the Ct (threshold cycle) value.
Ordering Information
| No. | Product Description |
| ENT-1111 | AccuPower® EV Real-Time RT-PCR Kit (96 tests) |
Related Products
| Cat. No. | MDx Diagnostic Kit |
| E71-1111 | AccuPower® Enterovirus 71 Real-Time RT-PCR Kit (96 tests) |
| Cat. No. | Instrument |
| A-2060 | Exicycler™ 96 Real-Time Quantitative Thermal Block |
| A-5050 | ExiPrep™16 Dx Automated Nucleic Acid Extraction System (16 tests) |
| Cat. No. | Nucleic Acid Extraction Kit |
| K-4471 | ExiPrep™ Dx Viral DNA/RNA Kit (96 tests) |
| K-4473 | ExiPrep™ Dx Viral RNA Kit (96 tests) |
Quality Assurance
Bioneer is the holder of Quality Management System Certificates for the following standards.
Contact Us
E-mail : sales@bioneer.com