We use cookies to give you the best online experience. By using our website you agree to our use of cookies in accordance with our Cookie Policy
AccuPower® BKV Quantitative PCR Kit
BK virus (BKV) was originally discovered and isolated from the urine of a renal allograft recipient. The source of BKV infection is due to decline in immunologic activity from either graft dysfunction and graft rejection in renal transplant recipients under immunosuppressed state. Detection of BKV has traditionally relied on tissue examination, but PCR is the best diagnostic test for use in human samples since it provides fast and accurate results and is highly sensitive and specific for quantification of BKV DNA.
Features and Benefits
- Simplified Procedure: Serum and urine samples can be directly used for the quantification of BKV viral load using ExiStation™ MDx System.
- Signature Convenience: All components for the assay are contained within a tube.
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
Specifications
| Specimen Type | Serum, urine |
| Kit Contents | PCR Premix, SPC 1~5, IPC, NTC, SL buffer, DEPC D.W. |
| Instrumentation | ExiStation™ Universal Molecular Diagnostic System or Exicycler™ 96 |
| Tests | 96 |
Performances
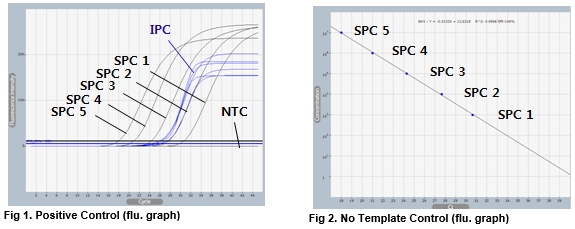
AccuPower® BKV Quantitative PCR Kit includes serially diluted Standard Positive Control (SPC) 1~5 for the quantification of BKV.
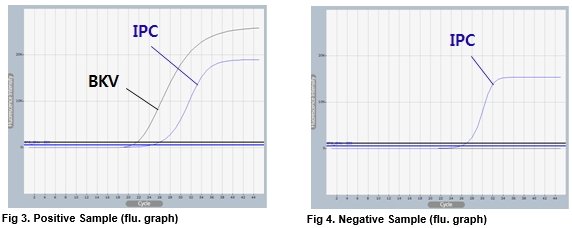
AccuPower® BKV Quantitative PCR Kit test results using clinical samples. The kit employs IPC in all wells to confirm correct PCR amplification.
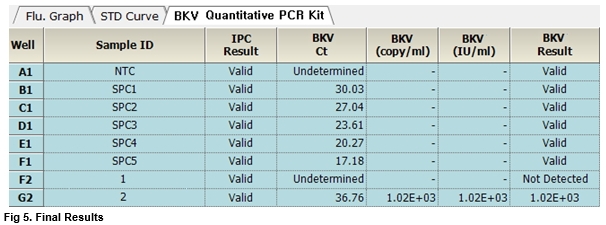
User centric ExiStation Manager software or ExiDiagnosis software automatically analyzes the test results based upon the Ct (threshold cycle) value.
Ordering Information
| Cat. No. | Product Description |
| BKV-1111 | AccuPower® BKV Quantitative PCR Kit (96 tests) |
Related Products
| Cat. No. | MDx Diagnostic Kit |
| CMV-1111 | AccuPower® CMV Quantitative PCR Kit (96 tests) |
| EBV-1111 | AccuPower® EBV Quantitative PCR Kit (96 tests) |
| Cat. No. | Instrument |
| A-2060 | Exicycler™ 96 Real-Time Quantitative Thermal Block |
| A-2200 | ExiStation™ Universal Molecular Diagnostic System |
| A-5050 | ExiPrep™16 Dx Automated Nucleic Acid Extraction System (16 tests) |
| Cat. No. | Nucleic Acid Extraction Kit |
| K-4471 | ExiPrep™ Dx Viral DNA/RNA Kit (96 tests) |
| K-4472 | ExiPrep™ Dx Viral DNA Kit (96 tests) |
Quality Assurance
Bioneer is the holder of Quality Management System Certificates for the following standards.
Contact Us
E-mail : sales@bioneer.com




