We use cookies to give you the best online experience. By using our website you agree to our use of cookies in accordance with our Cookie Policy
AccuPower® STI8A-Plex Real-Time PCR Kit
Sexually Transmitted Infection (STI) is an infection that can be transferred from one person to another by means of sexual contact, including oral sex, anal sex, and sharing sex toys. Chlamydia infection is a common sexually transmitted infection in human caused by the bacterium Chlamydia trachomatis. Neisseria gonorrhoeae is pathogenic to humans who are its only natural host, and it is responsible for the disease Gonorrhea. Symptoms of infection with N. gonorrhoeae differ, depending on the site of infection. Ureaplasma urealyticum is part of the normal genital flora of both men and women. It is found in about 70% of sexually active humans. Mycoplasma genitalium is an emerging sexually transmitted pathogen implicated in urethritis in men and several inflammatory reproductive tract syndromes in women. Depending on the disease, some untreated STIs can lead to infertility, chronic pain or even death. Early identification and treatment results in less chance to spread disease and for some conditions may improve the outcomes of treatment.
Features and Benefits
- Multiplex Detection: Simultaneous detection of 8 STI pathogens with STI8B-Plex Real-Time PCR Kit using ExiStation™ MDx System.
- Signature Convenience: All components for the assay are contained within a tube.
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
Specifications
| Specimen Type | Urine, vaginal swab, urethral swab |
| Kit Contents | PCR Premix, PC, IPC, NTC, DEPC D.W. |
| Instrumentation | ExiStation™ Universal Molecular Diagnostic System or Exicycler™ 96 |
| Tests | 96 |
Performances
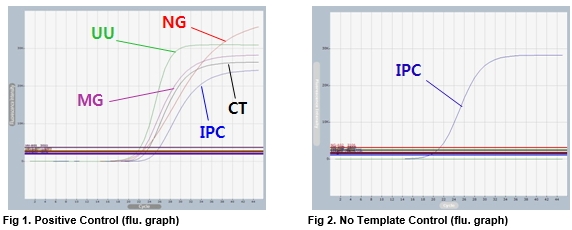
AccuPower® STI8A-Plex Real-Time PCR Kit includes Positive Control (PC), No Template Control (NTC) and Internal Positive Control (IPC) for accurate and reliable diagnosis of STI pathogens.
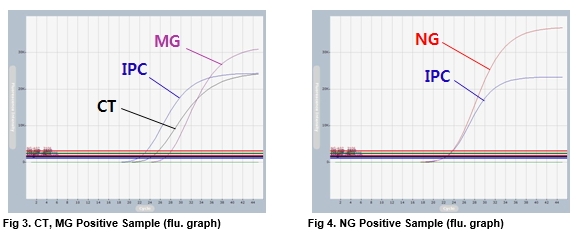
AccuPower® STI8A-Plex Real-Time PCR Kit test results using clinical samples. The kit employs IPC in all wells to confirm correct PCR amplification.
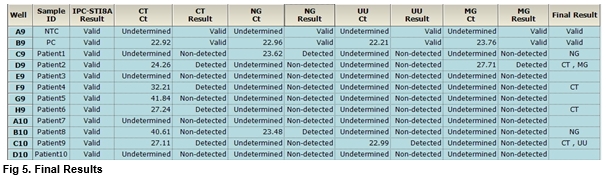
User centric ExiStation Manager software or ExiDiagnosis software automatically analyzes the test results based upon the Ct (threshold cycle) value.
Ordering Information
| Cat. No. | Product Description |
| STI8A-1111 | AccuPower® STI8A-Plex Real-Time PCR Kit (96 tests) |
Related Products
| Cat. No. | MDx Diagnostic Kit |
| CHT-1111 | AccuPower® CT Real-Time PCR Kit (96 tests) |
| STD2A-1211 | AccuPower® CT&NG Real-Time PCR Kit (96 tests) |
| STI8B-1111 | AccuPower® STI8B-Plex Real-Time PCR Kit (96 tests) |
| Cat. No. | Instrument |
| A-2060 | Exicycler™ 96 Real-Time Quantitative Thermal Block |
| A-2200 | ExiStation™ Universal Molecular Diagnostic System |
| A-5050 | ExiPrep™16 Dx Automated Nucleic Acid Extraction System (16 tests) |
| Cat. No. | Nucleic Acid Extraction Kit |
| K-4414 | ExiPrep™ Dx Bacteria Genomic DNA Kit (96 tests) |
Quality Assurance
Bioneer is the holder of Quality Management System Certificates for the following standards.
Contact Us
E-mail : sales@bioneer.com




