We use cookies to give you the best online experience. By using our website you agree to our use of cookies in accordance with our Cookie Policy
AccuPower® New Inf A(H1N1) & Inf A Real-Time RT-PCR Kit
Influenza occurs all over the world, with an annual global attack rate estimated at 5–10% in adults and 20–30% in children. In temperate regions, influenza is a seasonal disease occurring typically in winter months. The subtypes of influenza A viruses are determined by envelope glycoproteins possessing either haemagglutinin (HA) or neuraminidase (NA) activity. Influenza A (H1N1) virus emerged in 2009 is a new reassortment that has never before circulated among humans. This virus is not closely related to previous or current human seasonal influenza viruses.
Features and Benefits
- Signature Convenience: One-step RT-PCR premix type. All components for the assay are contained within a tube.
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
- Low coefficient of variation: Low coefficient of variation (CV) for between-day precision and inter-instrument trueness within 2.02 %
Specifications
| Specimen Type | Nasopharyngeal swab, BAL |
| Kit Contents | PCR Premix, PC, IPC, NTC, DEPC D.W. |
| Instrumentation | Exicycler™ 96 |
| Tests | 48 |
Performances
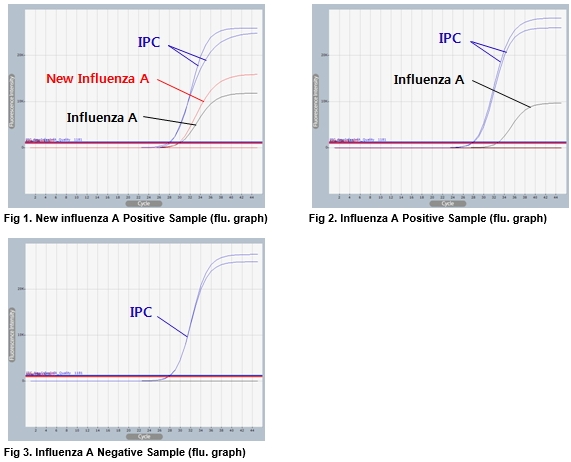
AccuPower® new InfA (H1N1) & InfA Real-Time RT-PCR Kit test results using clinical samples. The kit employs IPC in all wells to confirm correct PCR amplification. The test includes Positive Control (PC), No Template Control (NTC) and Internal Positive Control (IPC) for accurate and reliable diagnosis of influenza A (H1N1-2009, common).
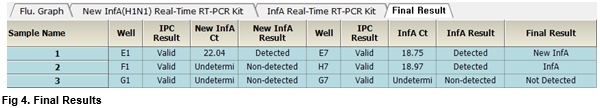
User centric ExiDiagnosis software automatically analyzes the test results based upon the Ct (threshold cycle) value.
Ordering Information
| Cat. No. | Product Description |
| SIA-1111 | AccuPower® new Inf A (H1N1) & Inf A Real-Time RT-PCR Kit (48 tests) |
Related Products
| Cat. No. | MDx Diagnostic Kit |
| IFA-1111 | AccuPower® Influenza A Real-Time RT-PCR Kit (96 tests) |
| SIV-1111 | AccuPower® new Inf A (H1N1) Real-Time RT-PCR Kit (96 tests) |
| Cat. No. | Instrument |
| A-2060 | Exicycler™ 96 Real-Time Quantitative Thermal Block |
| A-5050 | ExiPrep™16 Dx Automated Nucleic Acid Extraction System (16 tests) |
| Cat. No. | Nucleic Acid Extraction Kit |
| K-4471 | ExiPrep™ Dx Viral DNA/RNA Kit (96 tests) |
| K-4473 | ExiPrep™ Dx Viral RNA Kit (96 tests) |
Quality Assurance
Bioneer is the holder of Quality Management System Certificates for the following standards.
Contact Us
E-mail : sales@bioneer.com



