We use cookies to give you the best online experience. By using our website you agree to our use of cookies in accordance with our Cookie Policy
AccuPower® MP Real-Time PCR Kit

Mycoplasma pneumonia is a form of atypical bacterial pneumonia caused by the bacterial species Mycoplasma pneumoniae. In general, M. pneumoniae infection is a mild illness that is most common in young adults and school-aged children. The most common type of illness, that is caused by the bacteria, especially in children, is tracheobronchitis, commonly called a chest cold. Diagnosis of M. pneumoniae infections is complicated by the delayed onset of symptoms and the similarity of symptoms to other pulmonary conditions. Among other diagnostic tools, PCR is the most rapid and effective way to determine the presence of M. pneumoniae.
Features and Benefits
- Signature Convenience: Simplified procedure of sputum pretreatment with EZ Solution. All components for the assay are contained within a tube. Just add sample!
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
- Low coefficient of variation: Low coefficient of variation (CV) for between-day precision and inter-instrument trueness within 1.89 %
Specifications
| Specimen Type | Sputum, BAL |
| Kit Contents | PCR Premix, PC, IPC, NTC, DEPC D.W. |
| Instrumentation | ExiStation™ Universal Molecular Diagnostic System or Exicycler™ 96 |
| Tests | 96 |
Performances
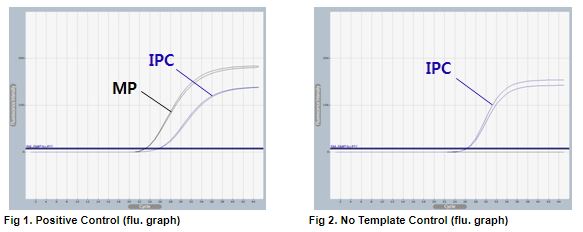
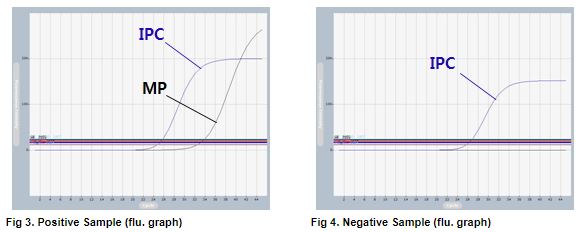
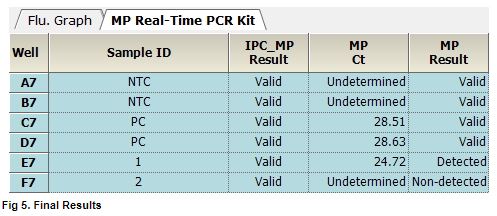
AccuPower® MP Real-Time PCR Kit includes Positive Control (PC), No Template Control (NTC) and Internal Positive Control (IPC) for accurate and reliable diagnosis of M. pneumoniae.
AccuPower® MP Real-Time PCR Kit test results using clinical samples. The kit employs IPC in all wells to confirm correct PCR amplification.
User centric ExiStation Manager software or ExiDiagnosis software automatically analyzes the test results based upon the Ct (threshold cycle) value.
Ordering Information
| Cat. No. | Product Description |
| MPN-1111 | AccuPower® MP Real-Time PCR Kit (96 tests) |
Related Products
| Cat. No. | MDx Diagnostic Kit |
| CPN-1111 | AccuPower® CP Real-Time PCR Kit (96 tests) |
| Cat. No. | Instrument |
| A-2060 | Exicycler™ 96 Real-Time Quantitative Thermal Block |
| A-2200 | ExiStation™ Universal Molecular Diagnostic System |
| A-5050 | ExiPrep™16 Dx Automated Nucleic Acid Extraction System (16 tests) |
| Cat. No. | Nucleic Acid Extraction Kit |
| K-4414 | ExiPrep™ Dx Bacteria Genomic DNA Kit (96 tests) |
| K-4418 | ExiPrep™ Dx Mycobacteria Genomic DNA Kit (96 tests) |
Quality Assurance
Bioneer is the holder of Quality Management System Certificates for the following standards.
Contact Us
E-mail : sales@bioneer.com