We use cookies to give you the best online experience. By using our website you agree to our use of cookies in accordance with our Cookie Policy
AccuPower® MERS-CoV (upE&ORF1a) Real-Time RT-PCR Kit

Middle East respiratory syndrome (MERS) is a viral respiratory illness caused by a coronavirus called MERS-CoV. It was first reported in Saudi Arabia in 2012 and has spread to other countries including South Korea. Camels and bats are suspected to be the primary source of the infection for humans. MERS causes high fever, cough, and severe shortness of breath, and can also leads up to kidney failure and even death in patients who had a history of other medical conditions. There are currently no vaccines for MERS and no treatments available to cure the infection.
Features and Benefits
- Signature Convenience: One-step RT-PCR premix type. All components for the assay are contained within a tube. Just add sample!
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary Dual HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
Specifications
| Specimen Type | Sputum, BAL and throat swab |
| Kit Contents | PCR Premix, PC, IPC, NTC, DEPC D.W. |
| Instrumentation | ExiStation™ Universal Molecular Diagnostic System or Exicycler™ 96 |
| Tests | 96 |
Performances
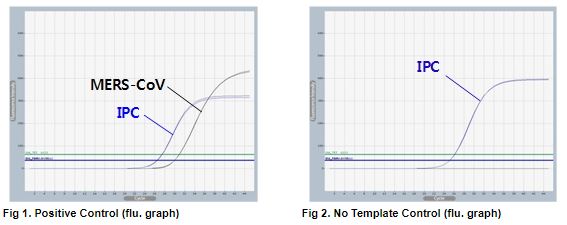
AccuPower® MERS-CoV Real-Time RT-PCR Kit includes Positive Control (PC), No Template Control (NTC) and Internal Positive Control (IPC) for accurate and reliable diagnosis of MERS-CoV upstream of E gene (upE).
Ordering Information
| Cat. No. | Product Description |
| COV-1111 | AccuPower® MERS-CoV Real-Time RT-PCR Kit (96 tests) |
Related Product
| Cat. No. | MDx Diagnostic Kit |
| COV-1112 | AccuPower® MERS-CoV (upE & ORF1a) Real-Time RT-PCR Kit (CE) |
| Cat. No. | Instrument |
| A-2060 | Exicycler™ 96 Real-Time Quantitative Thermal Block |
| A-2200 | ExiStation™ Universal Molecular Diagnostic System |
| A-5050 | Exicycler™ 16 Dx Automated Nucleic Acid Extraction System |
| Cat. No. | Nucleic Acid Extraction Kit |
| K-4473 | Exicycler™ Dx Viral RNA Kit (96T) |
| K-4475 | Exicycler™ Dx Sputum Viral RNA Kit (96T) |
Quality Assurance
Bioneer is the holder of Quality Management System Certificates for the following standards.
Contact Us
E-mail : sales@bioneer.com